
Pharmaceutical Methods
Publishing Quality Research & Reviews

Pharmaceutical Methods
Publishing Quality Research & Reviews
Mini Review - (2022) Volume 13, Issue 1
Received: Feb 25, 2022, Manuscript No. PHMETHODS-22-55525; Editor assigned: Feb 28, 2022, Pre QC No. PHMETHODS-22-55525 (PQ); Reviewed: Mar 14, 2022, QC No. PHMETHODS-22-55525; Revised: Mar 21, 2022, Manuscript No. PHMETHODS-22-55525 (R); Published: Mar 30, 2022, DOI: 10.35248/2229-4708.22.13.224
The protocols are important to assess the factors determining the features of a clinical study under their items the protocol clarifies the methods, the data setting and management, and the administrative and introductory information. Several issues may be caused if any of these elements are missing. Firstly, trial couldn’t be approved without any of these elements, secondly, they’re part of the clinical study and their absence may create confusion during the conduction of the study, in third instance the method sections set the methods to take the trial itself. It is very important to consider some statistical features in the onset of a trial, and indeed accuracy/precision and overall measurement scales are necessary to collect data and then evaluate the error. There are two types of errors in testing a statistical hypothesis: the type I error accept a false hypothesis as correct, and type II error is on the contrary the failure to reject a true hypothesis as incorrect. It’s important to calculate the hypothesis to evaluate the probability we could have a positive or a negative found in the sample data. An estimate may be done on the effect size, which is the quantity of the force of a phenomenon. Another feature is the calculus of the sample size done previously starting the trial. Without a correct number of participants, the study itself wouldn’t give the appropriate result. “Number needed to treat” is an advantaging method to deal with adverse event such as death. The main issue with which statistician may occur is the number of patients available to participate to the trial. All these facts are connected one by one, and they reflect the statistical arrangements need to be considered to produce the correct data outcomes.
Drug metabolism, Alkaline phosphatase, Creatinine, Clinical trial, Gastrointestinal bleeding.
This article aims to outline main protocol preparation characteristic correctly. The description based on five steps i.e., content, patient’s inclusion/exclusion criteria, ethical consideration, clinical trial governance principles, and data management. Advise composes this overview aims to review the protocol path to researchers aim to obtain the clinical trial approval. The biological sample analysis result is very important to determinate the conditions of health in the candidate. The most important seem to be the one related to the drug metabolism, so that protein urea, creating clearance, and alkaline phosphatase represent renal function; and transaminases represent a normal liver function.
A protocol is the statement that clarifies the assessment of a clinical trial. The protocol clarifies the objectives, the design, the procedure, the statistical analysis, and the organisation of a clinical study and it is composed by several sections.
Step 1
Administrative information:
•The title to identify the study
• Trial registration to register the name
• Protocol version to identify date and version
• Funding to set the financial and material support
• Roles and responsibility to assign the roles of protocol contributors
• Name and sponsor contact details
• Role of the sponsor
• Background and rationale to clarify the reason to install the trial
• Explain the choice of comparators
• Specify the objectives
• The trial design description should include the type of trial, the allocation ratio and the framework
Strategies:
• Eligibility criteria
• Interventions details
• Outcomes
• Time program of the participants
• Sample size
• Recruitment procedure
Allocation:
• Sequence generation
• Allocation mechanism
• Implementation
• Blinding or masking
Data collection and management:
• Data collection methods
• Data management is a program for data collection and storage
• Statistical methods to analyse the results
Monitoring:
•The data monitoring is a resume of the role of the data monitoring committee (DMC)
•The monitoring harms
• The auditing procedures
Ethics and dissemination:
• The research ethics committee and institutional review board (REC/IRB)
• Protocol amendments description
• Notice the absence of a participant
• Declaration of interests for the financial interests
• Ancillary and post-trial care set the assistance
• Dissemination policy is the communication of the results to patients, investigators, and regulatory authority
Appendices:
• Informed consent materials
• Handling biological specimens’ policy
Step 2
Inclusion criteria:
These are the roles with which participants are recruited to participate in a trial under their physical conditions or personal peculiarities. As it follows a list of instances may include a patient in a clinical study such as Poor left ventricular function (EF ≤ 35%), Histological proven stage III (stage T2, T3 or T4) and stage II (any one or more of the following stages T4, lymphatic invasion or vascular invasion, and peritoneal involvement) colorectal cancer (expected ratio 70%: 30%). Participants could be recruited during Stage II, T3 only when they have a week prognosis, Patients must have resected the whole primary tumour and the disease need to be completely cured, Patients must be randomised to start treatment with a minimum of 4 weeks and maximum of 71 weeks after surgery, WHO Performance Status 0 or 1, Male or female patients age must be 18 years old or more, The expectations of life must be equal or major of 5 years [1].
Exclusion criteria:
These are the considerations must be made to exclude specific patients from being enrolled in a trial. These reasons are ethically evaluated and they consider a wide range of difficulty and issues in managing patients during study include Myocardial infarction minor 4 weeks prior to randomisation, The treatment with other therapeutic agents must be interrupted 4 weeks before the beginning of the study to avoid interactions, Moderate or severe kidney damage and dialysis (creatinine clearance value about 30 ml/min), Women during pregnancy, Life expectancy minor than 1 year. Gastrointestinal bleeding or other dysfunctions of the gastrointestinal tract like chronic inflammatory bowel disease, mal-absorption syndrome, diarrhoea or could influence the adsorption of oral medications. Central nervous system illnesses or psychiatric disability evaluated by the investigator to be enough significant to preclude informed consent. Female candidates must have started the menopause period 12 months before to be considered out of risk out pregnancy.
Whether a woman is pregnant she cannot participate to the trail because their admission would be ethically incorrect; the same consideration could be done if a mind-unable/brain-ill candidate would be admitted to a clinical study [2]. The four definitions are the mainly used to identify the binary data of a trial in statistic include the relative risk measures the magnitude of an association between an exposed and non-exposed group. It describes the likelihood of developing disease in an exposed group compared to a non-exposed group, the multivariate risk reports the happening at the same time of many events; differently from the univariate risk used to calculate the incidence of one event at the time, the odds ratio is the relationship between the frequency with which an event happens in a group of patients and the frequency with which the same event happens in patient control group, the p-value represent the meaningfulness level, therefore it is evidence of measure against a nothing hypothesis, the random variable is a value that can change due to peculiar chances, the data in a cohort study wants to evaluate the risk relative risk would be very appropriate to measure the likelihood of the risk factor between patient and control and the data in cross sectional study aim to measure the prevalence of phenomena in the study, so that a univariate risk and multivariate risk fits with this type of study.
In a randomized trial the random variable is an important factor to evaluate the part of data deviate from the samples data. In each of the cases already mentioned the p-value is applicable to measure the data gets out of the standard volume of measurements and it can be also related to the original hypothesis [3].
Step 3
The research is a systematic investigation to evaluate the facts in a scientific way, it develops new skills of knowledge on a particular inquiry; uncertainty is a peculiarity of research, and it is related to the outcomes and to the inquiry. Clinical trial involves human subjects with specific ethical issues. Furthermore, Tom Beauchamp and James F. Childress invented in 1979 four ethical roles:
• Autonomy (avoiding obligation)
• Non-maleficence (avoiding harm)
• Beneficence (balancing benefits/risks)
• Justice (benefits/risks distributed)
The inclusion/exclusion criteria are set on three items: identification of potential patients, approach, and recruitment. The ethical issues that arise from this procedure include the data protection laws, and privacy policies guaranties the respect of the data to identify a potential participant and the coherence to undertake a trial is an important aspect and it must be carefully treated [4].
The recruitment of the population during a trial involves some ethical aspects; therefore, ethical issues may be resolved respecting the roles listed in the following section:
• Co-morbidity: a previous unknown condition of health allows the investigator to exclude the participant from the study
• Previous or current treatment: The assumption of a drug or a surgery can preclude the participation to the study
• Pregnancy, contraception, and lactation may be the reason of exclusion from a trial
• Personal characteristic: Age, sex, and ethnicity
• The absence of diagnostic exams is a fundamental pre-requisite for attending in a trial [5].
Step 4
It is very important to consider governance to require trial approval may delineate two general principles i.e., the investigator should maintain the neutrality between standard care and research and the regulatory authorities have developed procedures to help identification of the problems in the way to arrange practice work skills.
The Principles of ICH (International Conference on Harmonization) in GCP (Good Clinical Practice) are resumed as it follows:
• Clinical trials should be conducted according to the Declaration of Helsinki who’s the ethical roles are to be followed to conduce a trail.
• Before a trial starts, the benefit-risk balance should be assessed anticipatory to qualify the benefit for the patient exposed to the risk. A trial should be started and brought only if the evaluation of the benefits justifies the risks anticipatory.
• The rights, safety of the patient is more important than the interests of science itself and of the economic interest of the funder and/or sponsor.
• The data collected in preclinical and/or clinical study should be enough to support the application of the clinical trial.
• Clinical trials application must be supported by detailed protocol.
•The protocol is the governance for a clinical trial, and it is submitted to the competent authority for: a) institutional review board (IRB) b) independent ethics committee approval (IEC) c) and receive the approval of a favourable opinion.
• The medical care responsibility must be assigned to qualified physician.
• Each professional figure involved in a trial must be trained and qualify.
•The informed consent must be distributed obligatory before the clinical trial starts.
• All clinical trial data must be handled with precision and accuracy in the way not to cause a lack of reporting and/or interpretation.
• The respect of the privacy and confidentiality must be followed by the regulatory requirements [5-7].
Step 5
The clinical data management is an important feature to ensure quality data. They are collected by the staff on the paper case report form (CRF) or on the electronic case report form (eCRF).
Strategies for data management are:
• Data governance, guideline, and standardizing data
• Data analysis sets the sample size; it explains how the patients are evaluated to be necessary to lead the trial
• Database management is the trial data administration
• Data security management ensures that the security policy is respected
• Data quality management proofs the quality of the data
• The data manager evaluates the data modification, publishing, and transfer data (Fig. 1) [8,9].
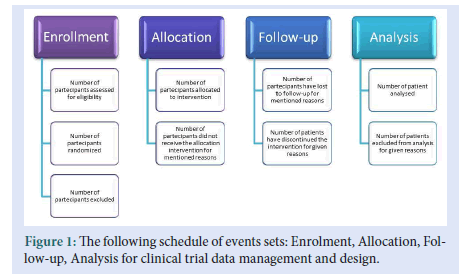
Figure 1: The following schedule of events sets: Enrolment, Allocation, Follow-up, Analysis for clinical trial data management and design.
Drug allergy is an important factor to be considered because a patient affected by the symptoms of an allergy might interrupt the participation to the trial. The instruction allows filling a clinical trial protocol statement correctly. These five steps allow approaching protocol preparation promptly. As they fulfil necessary concepts to protocol principles understanding by each main part. The ethical principles are a guidance for clinical studies that involves human beings. The autonomy requires the voluntaries of the patient to participate to the trail without being forced. The non-maleficence is the principle determinates the risk in which the patients occur. Inclusion and exclusion criteria are the roles to recruit the patients and it should remain constant during the clinical study trial time. The changes are possible only during long term trial and they are based on analysis might suggest a change of these criteria.
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
Pharmaceutical Methods received 3403 citations as per google scholar report