
Pharmaceutical Methods
Publishing Quality Research & Reviews

Pharmaceutical Methods
Publishing Quality Research & Reviews
Review - (2022) Volume 13, Issue 1
Received: Jan 17, 2022, Manuscript No. PHMETHODS-22-51680; Editor assigned: Jan 20, 2022, Pre QC No. PHMETHODS-22-51680 (PQ); Reviewed: Feb 03, 2022, QC No. PHMETHODS-22-51680; Revised: Feb 10, 2022, Manuscript No. PHMETHODS-22-51680 (R); Published: Feb 18, 2022, DOI: 10.35248/2229-4708.22.13.225
Objective: Pregabalin (PGB) is an antiepileptic drug, named (S)-3-(aminomethyl)-5-methyl hexanoic acid. Since the discovery, PGB has remained the main representative drug from the antiepileptic category, due to its unique mode of action which concerns the treatment of painful diabetic neuropathy postherpetic neuralgia by lagging down the impulses in the brain which causes seizures.
Chemically, PGB shows amphoteric (zwitterionic) properties and the presence of a chiral center reflects the comparatively high biological activity of one of its isomers. Analytically, the ring closure and ring-opening of PGB depend on the solubility and the method or instrument adopted. The analytical techniques in pharmaceuticals are used for analysis and drug monitoring remains important in perceiving factors like bioavailability, bioequivalence, and therapeutic monitoring.Due to all mentioned features, it becomes necessary to compile the various analytical methods that have been reported in the literature for its analysis. The present critical review assesses the compilation of relevant articles that have already been published in recent ten years, describing analytical methods like UV, HPLC (single and combined dosage form), HPTLC, LC-MS/MS, and GC/ MS method for the same.
Conclusion: The present comprehensive review disclosed a summary of pharmacokinetics-dynamics, pharmaceutical preparations, and analytical methods of bulk drug and biological matrices since the time of discovery.
Pregabalin, RP-HPLC, LC-MS/MS, HPTLC, GC-MS, Pharmacokinetic, Pharmacodynamics, Synthesis.
PGB: Pregabalin; NT: Neurotransmitter; GBP: Gabapentin; VGP: Vigabatrin; TOP: Topiramate; BLF: Baclofen; VGSCs: Voltage-Gated Sodium Channels; VGPCs: Voltage-gated Potassium Channels; VGCCs: Voltage-Gated Calcium Channels; DDQ: 2,3-Dichloro-5,6-Dicyano-1,4-Benzoquinone; TCNQ: 7,7,8,8-Tetracyanoquinodimethane; ALA: Alpha-Lipoicacid; λmax: Lamda Max; RF: Retention Factor; RT: Retention Time; FR: Flow Rate; UV-VIS: UV-Visible Spectrometry; HPLC: High-Performance Liquid Chromatography; RP-HPLC: Reverse- Phase High-Performance Liquid Chromatography; HPTLC: High Performance Thin Later Chromatography; LC-MS/MS: Liquid Chromatography-Mass Spectrometry/ Mass Spectrometry; GC/GC-MS: Gas Chromatography/Gas Chromatography-Mass Spectrometry; DCC: Dicyclohexylcarbodiimide; NHS: N-Hydroxysuccinimide; EDC:1-ethyl- 3-(3-Dimethylaminopropyl) Carbodiimide; ECF: Ethyl Chloroformate; MCF: MethylChloroformate; IBCF: Isobytyl- Chloroformate; OPA: Orthophosphoric Acid; IUPAC: International Union of Pure and Applied chemistry; CNS: Central Nervous System; ATP: Adenosine Triphosphate; Ca2+: Calcium2+; MIP: Indian Pharmacopoeia; Cm: Centimeter; mm: Millimeter; nm: Nanometer; μl: Micro liter; μg: Microgram; NaOH: Sodium hydroxide; ACN: Acetonitrile; DW: Distilled water; LOD: Limit of Detection; LOQ: Limit of Quantification; ®: Registered Trademark.
FNeuropathic pain is the result of hyper-excitability of neurons damaged areas of nerves which are similar to that of convulsion [1]. Drugs like Gabapentin (GBP) and its analog Pregabalin (PGB) were designed against pains associated with trigeminal neuralgia and diabetic neuropathy [2]. Both analogs GBP and PGB are essential in treatment because of their minimum drug interactions and side effect [3]. Most of the drugs of the epilepsy section work on varied mechanisms and so considered to be broad-spectrum drugs [4]. The basic mechanism of antiepileptic drug based on the most important inhibitory neurotransmitter (NT) of the Central Nervous System (CNS), γ-aminobutyric acid (GABA) enhancement. Modulation of voltage-gated ion channel and attenuation of glutamate-mediated NT in the brain is considered as other mechanisms for drugs like PGB[5,6]. The GABA is considered to exert major and rapid inhibitory action from all classes of NT in the brain. It is believed that GABA is ionotropic [7] and metabotropic [8] in its sub-forms. The GABA-A and GABA-C are considered ionotropic while GABAB is metabotropic [9,10]. GABA-A is the preferred target for anticonvulsant drugs like PGB and GBP [11,12]. Moreover, GABA-B receptors with a subunit of GABA-B RS are also abundant at the spine and dendrites, where they are influencing synaptic and dendritic functions, result in modulating calcium (Ca2+) signals[13]. Considering another mechanism of modulation of voltage-gated ion channel (Ca2+, Na+and K+), the calcium channel plays a vital role in regulating Ca2+ signaling united with the action of PGB. Such channels are also known as voltage-gated calcium channels (VGCCs) [14]. For the basic anticonvulsant drugs like phenytoin, the preferred target is voltage-gated sodium channels VGSCs) at the presynaptic nerve terminal of excitatory glutamate receptors[ 15]. These drugs cause highly neuronal firing when binding to an inactivation gate nearby resulting inactivation of VGSCs. The intimately associated voltage-gated K+ channels with the membrane repolarization process are again considered to be important for drugs like Levetiracetam[16,17]. The drug PGB is structurally related to both the inhibitory NT GABA and GBP. Chemically PGB is 3-(aminomethyl)- 5-methyl hexanoic acid or 4-amino-3-(2-methyl propyl)butanoic acid with molecular formula C8H18O2N, melting point 186-188 ºC and the molecular weight 159.23 g Mol-1 [18,19]. The PGB is available in the enantiomeric form (R) and (S) (Fig. 1) where the (S)-enantiomer is approximately 10 times more active than the (R)-enantiomer[20]. The compound has one stereogenic center and in preclinical studies, there was no indication of racemization of PGB S-enantiomer to the R-enantiomer [21]. The approved product LYRICA®(USP 6,197,819 B1-2001) capsules contain 25, 50, 75, 100, 150, 200, 225 or 300 mg of PGB. The LYRICA® with active ingredients contains S-(+)-4-amino- 3-(2-methyl propyl) butanoic acid [22]. PGB is a potent ligand of alpha-2-delta subunit of voltage-gated calcium channel in the central nervous system which shows analgesic, anticonvulsant and anxiolytic activity [23]. It is a structural analog, but functionally dissimilar, of naturally occurring transmitter GABA (γ-aminobutyric acid). It is generally used for epilepsy, neuropathic pain, and anxiety condition. It is soluble in an aqueous solution and partially soluble in nonpolar solvents like DMSO, ethanol, DMF. It’s a crystalline substance that occurs in a single morphic form and non-hygroscopic. It is thermally stable and not solvated [24]. Aside from the chemical, pharmacodynamic, and pharmacokinetic parts, which are older but accurate, the manuscript is being designed using the most recent spectroscopical data available from 2010 to 2021, as well as the LYRICA patent (2005) (Fig. 1).
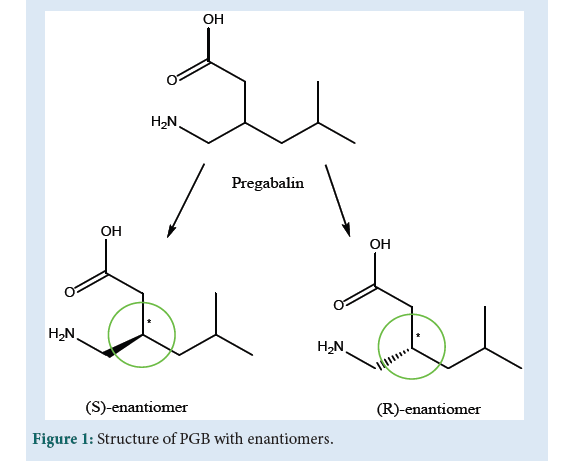
Figure 1: Structure of PGB with enantiomers.
Synthesis
PGB the best drug for the treatment of epilepsyis available in the enantiomeric form. As discussed, the S-(+) enantiomer is more potent than the R-(-) enantiomer and hence the synthesis of more active enantiomer or separation of enantiomeric form remains in focus during the designing of PGB. The schemes describe a few crucial industrial and patented methods to synthesize the PGB.
As per Fig. 2, the patented method of synthesis of PGB includes chiral oxazolidinone alkylation (asymmetric) chemistry. The chloride derivative (1.2) of (1.1) was used for acylation of the anion of chiral (1.3) to get (1.4) which was then alkylated with benzyl bro¬moacetate to produce (1.5) with greater than 95% enantio¬meric excess. The chiral auxiliary was treated with lithium hydroxide/hydrogen peroxide followed by sodium bisulfite to produce acid (1.6). The resulting acid was reduced to alcohol (1.7) using boranedimethyl sulfide. The obtained (1.7) was converted to the tosylate derivative using tosyl chloride in pyridine to produce (1.8) and then to azide (1.9) derivative by using sodium azidein dimethyl sulfoxide. Finally, the hydrogenation of azide in the presence of palladium on charcoal, catalyst gives PGB [25] (Fig. 2).
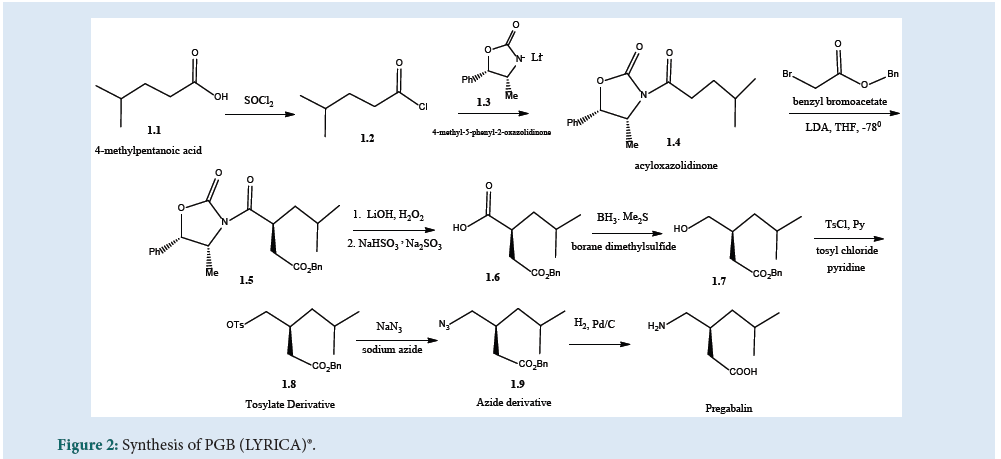
Figure 2: Synthesis of PGB (LYRICA)®.
The amino acid (2.1) in Fig. 3 can be converted to get (2.2) by treating sodium nitrite and sodium bromide in dilute acid. The retention of configuration can be seen in the proceeded reaction. In another step, the bromo acid was esterified using t-butyl acetate to produce (2.3). The compound (2.4) can be produced by displacement of bromide with diethylsodiomalonate, which was then hydrolyzed to the acid (2.5). Further, the chiral lactone (2.6) can be produced by reduction with boranedimethyl¬sulfide. The ring openings of the lactone with trimethylsilyl iodide produce (2.7) which gives (2.8) in treatment with sodium azide. At last, treatment of azide via hydrolysis of acid and hydrogenation produces PGB[26] (Fig. 3).
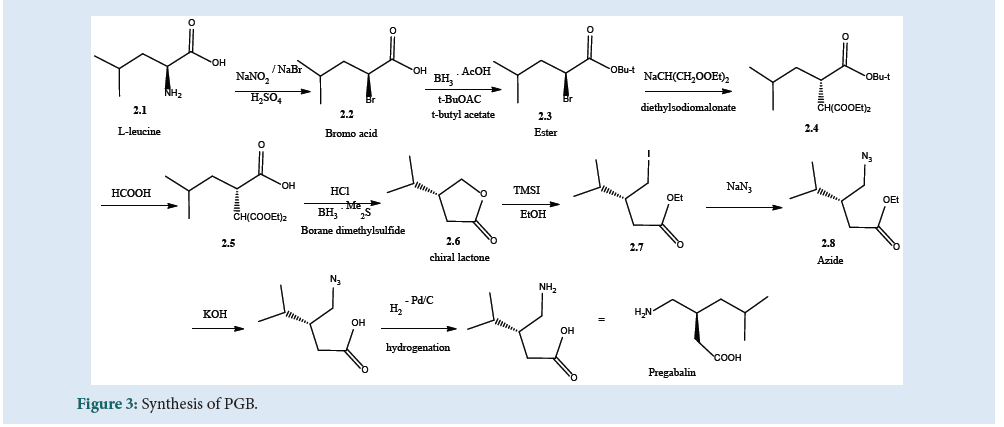
Figure 3: Synthesis of PGB.
Thesynthesis of PGB can be carried out by Stobbe condensation (Fig. 4) by utilizing (3.1) and (3.2) using di-n-propylamine/acetic acid as a catalyst to produce (3.3). Then, the hydro-cyanation product (3.4) can be obtained to get the racemic compound (3.5). Further, by hydrolysis and decarboxyl¬ation the final product of PGB can be obtained[27] (Fig. 4).
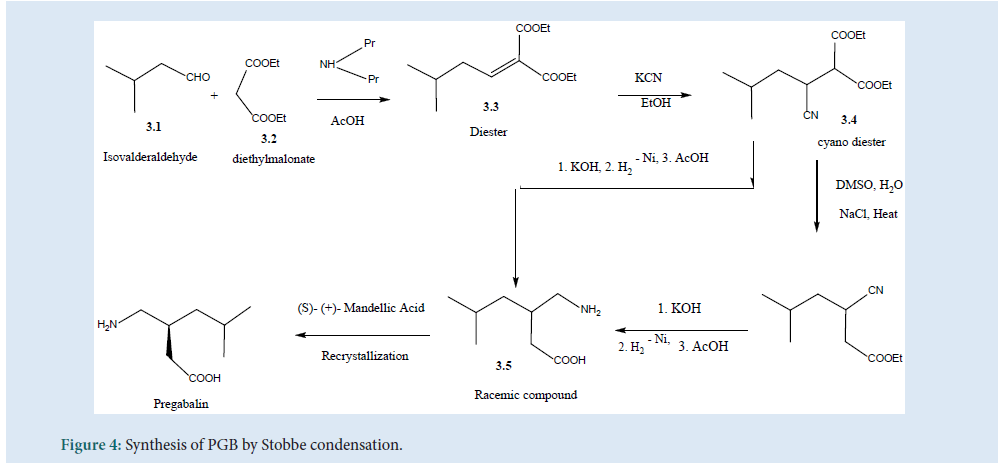
Figure 4: Synthesis of PGB by Stobbe condensation.
The synthesis of enantio-selective PGB can be carried out by the introduction of a nucleophile in β-position of a ά, β-unsaturated carbonyl using Iminium catalyst (4.3). The proclivity of catalyst (4.3) with (4.1) and (4.2) was used in the three-step synthesis of PGB[28] (Fig. 5).
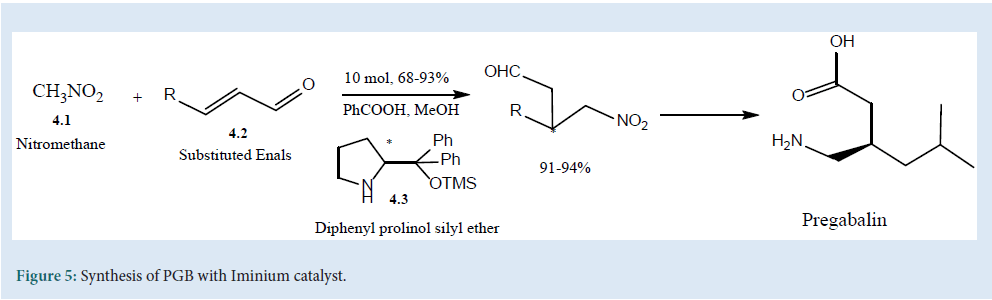
Figure 5: Synthesis of PGB with Iminium catalyst.
It describes the enantio-selective synthesis of PGB with a single chlorogenic center using enantio-selective hydrogenation of (5.1). The reaction carried out at 55oC in methanol using (5.2) asapre-catalyst to get (5.3) a high enantio-selective compound. Finally, the reaction scaled up with hydrogenation of nitrile to amino-group over a heterogeneous nickel, catalyst to obtain PGB [29] (Fig. 6).
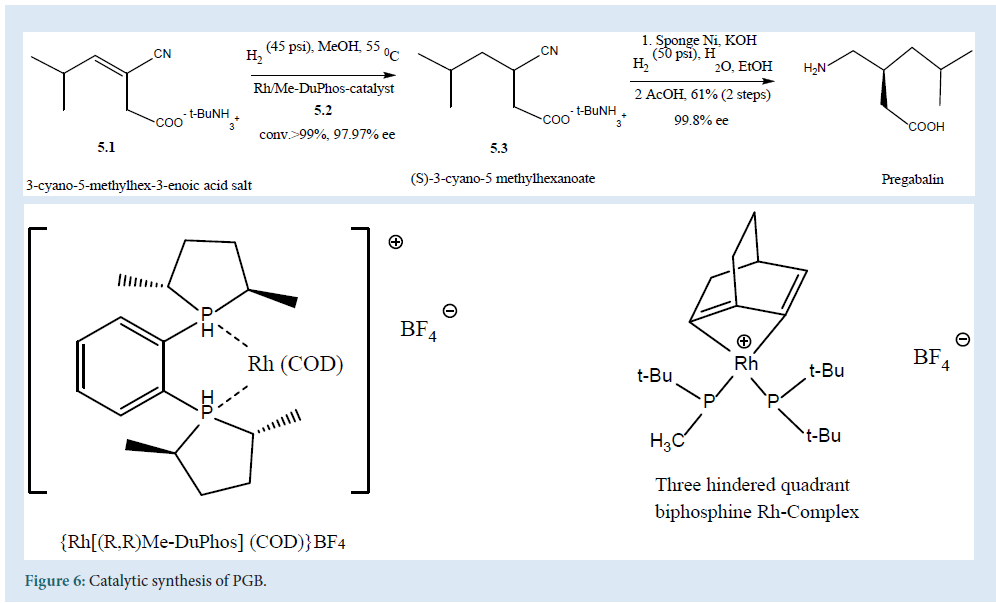
Figure 6: Catalytic synthesis of PGB.
Mechanism of action
PGB considerably resembles the (GBP) and accepted for the treatment of neuropathic pain since 2004. They are considered derivatives of inhibitory GABA. The evolution of PGB was coming into existence to suppress the anti-spastic effects of GBP. The mechanism of action of both the drugs used to demonstrate the efficacy in the treatment of both seizure disorders and pain syndromes. From the various mechanisms of actions of PGB, the action of voltage-gated sodium channels (VGSCs), voltage-gated potassium channels (VGPCs), and voltage-gated calcium channels (VGCCs), the action of VGCCs are considered as the primary mechanism. In the recent literature, it was found that neither PGB nor GBP affects sustained repetitive firing in the spinal cord or cortical neurons following acute exposure. The inadequacy of action at batrachotoxin binding sites in rat suggests that blocking VGSCs rarely contribute to the action of PGB [30,31].Considering the action of VGPCs, in a study, it was concluded that enlarging the exposure of PGB produces a late-onset allosteric enhancement of VGPCs in dorsal root ganglion neurons in rats. The action may be due to the activation of a specific protein named Kinase-A[32] (Fig. 7).
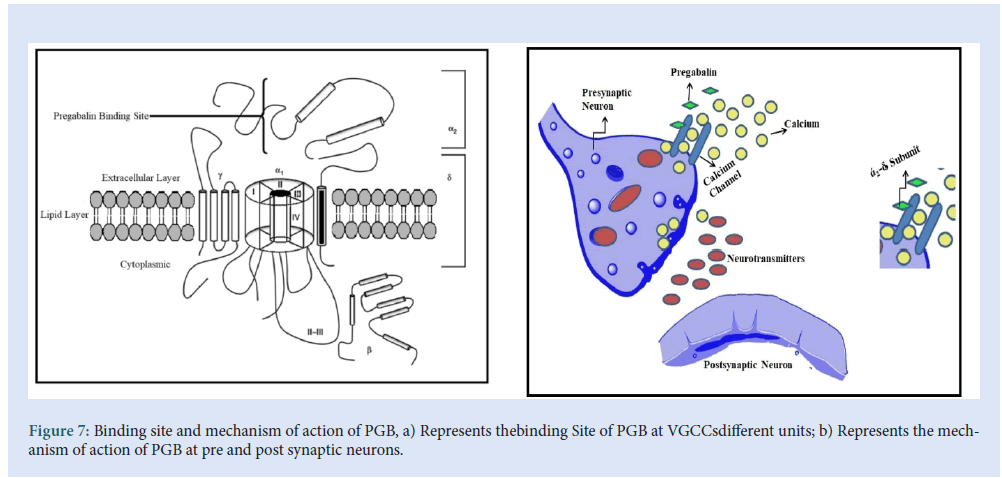
Figure 7: Binding site and mechanism of action of PGB, a) Represents thebinding Site of PGB at VGCCsdifferent units; b) Represents the mechanism of action of PGB at pre and post synaptic neurons.
PGB shows high-affinity binding to the α2δ subunit of the P/Q type of voltage-gated channel. The voltage-gated calcium channel is closed to resting membrane potential, the depolarization by action potential causes the channel to open which leads to the entry of Ca2+into the cell axonal membrane depolarizes when the action potential travels down to the neuron. When a voltage-gated calcium channel opens which causes an intrinsic current, the NT releases it from the synaptic vesicle and the multiplication of neurotransmission. In the presence of Ca2+exocytosis of NT and membrane fusion occurs [33,34].
PGB targets the voltage-gated calcium channel which consists of four subunits (Fig. 7a). The α1 subunit is a transmembrane array from a pore through which Ca2+ enters into the cell. The α2δ subunit contains δ protein linked by a disulfide bond to α2 protein, which has a higher affinity for the PGB binding site. The β subunit is intracellular and it modifies the functioning of the α2δ subunit, while the γ subunit is a glycoprotein that Inline in the cell membrane as shown in Fig. 7b [35- 38].
PGB pharmacokinetics
PGB is quickly absorbed and shows linear pharmacokinetics after oral administration. Its oral bioavailability is ≥ 90% peak plasma concentration arises 1 hr after oral administration and constant concentration achieve within 24-48 hrs. 20-25% peak plasma level decreases by the food intake and increase time to peak level by 3 hrs [39-41].This study includes single-dose and multi-dose tolerance studies. PGB has a comparatively short half-life which has a volume of distribution of 0.5 L kg-1 which does not bind to plasma protein. Pharmacokinetics investigation of clinical studies shows that pharmacokinetics PGB was not significantly influenced by sex or race [42,43].
Adsorption
PGB is quickly and widely absorbed after oral administration in the fasted state which shows the maximum plasma concentration after 1 hr in single or multiple doses and steady-state has been obtained within 24-48 hours after repeated dosing [44]. These fast absorption properties reflect the observed onset of efficacy as soon as a weak one in clinical trials performed in patients with partial epilepsy.
Distribution, metabolism, and elimination
PGB is a substrate of the system L carrier is capable of the transport of large amino acids across the brain and the gut. Coherent with this PGB can speedily cross the blood-brain barrier conducted in mice during the preclinical studies which are an obvious advantage for a drug that increases the CNS activity [45,46]. The biotransformation studies of PGB state that it gets absorbed almost completely (98%) and only approx. 2% of the dose can be recovered from the urine (as metabolites). This is considered as a minimum or negligible metabolism where PGB excreted unchanged in the urine. The principal metabolite obtained was N-methylpregabalin [47,48] (Fig. 8).
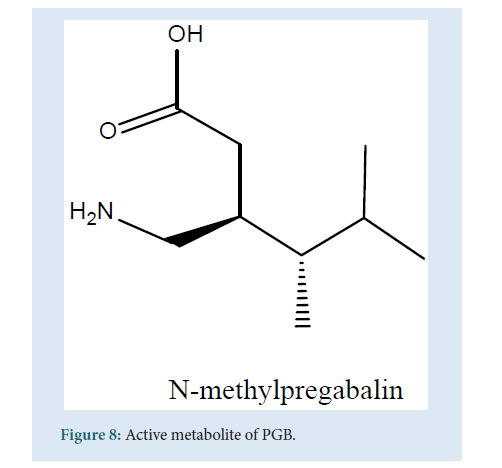
Figure 8: Active metabolite of PGB.
Due to the negligible amount of metabolite, it does not inflict hepatic metabolism or impaired liver functions and does not cross or restrict enzymes like the cytochrome P450 system. PGB could not bind to the plasma protein and also doesn't inhibit drug metabolism in vitro that’s why PGB is improbable to cause or subject to pharmacokinetic drugdrug interaction and the anticipation that has been proved in clinical pharmacokinetic studies [49-51].
Physicochemical properties
PGB (trade name-LYRICA®) exists in white to off-white crystal structure and single morphic form. The compound was found to be non-hygroscopic, non-solvated, stable, and water-soluble. In water/aqueous medium solubility was >30 mg ml-1 at RT. The pH of the drug ranges from 1 to 13 [52].The boiling point of PGB was found to be 274 ± 230 Cat 760 mm Hg [53] and thesolubility was observed in the water freely and also in basic and acidic solutions [54]. The vapor pressure was observed as 2.02 × 10-9 mm Hg at 25°C. The LogP value was observed as log Kow=-1.78 with optical Specific rotation as +10.52 degD-1 (c=1.00 in water) and Dissociation Constants-pKa1=4.2 and Pka2 of 10.6 [55- 57]. The log of the partition coefficient (n-octanol/0.05 M phosphate buffer) at pH 7.4 was -1.35. Henry's Law constant=6.89 × 10-11 atm-cu m mol-1 at 25°C[58].
Analytical accounts on PGB
Although drug analysis is an important method need to regulate in all the stages during development, from designing to the post-marketing phases, but here in the article we focus mainly on the phases of analytical development and pharmacokinetics investigation [59-62]. Therefore, an abundance of data related to drugs may be obtained through analytical techniques, including data such as physical and chemical stability of the drug, bioavailability, and bioequivalence of the drug, design of the dosage form, quantification, and identification of impurities in the marketed product, and the quantification of the drug content [63-65]. In addition, therapeutic drug monitoring was also performed using analytical techniques through the determination and investigation of the pharmacokinetic parameters of all the methods available, HPLC-based methods [single (Table 1) and combination with PGB (Tables 2 and 3)] have mostly used techniques for pharmaceutical analysis [66-81]. As PGB was easily soluble in water and at room temperature, ring closure does not occur; HPLC [82-94] is the most preferred method for its analysis. Several other methods like HPTLC [95-98], Ultraviolet- Visible [80,81] have been used for the determination of PGB in bulk and pharmaceutical formulation (Table 1).
| Sr. No | Matrix | Method | Detection (λmax) nm |
Solvent | Linearity | LOD and LOQ |
Ref | |
|---|---|---|---|---|---|---|---|---|
| 1 | Bulk and Pharmaceutical Dosage form |
UV spectrophotometric | 210 nm | Double DW |
6-14 μg/ml |
2.457 mg/ml 7.448 mg/ml | [71] | |
| 2 | Pharmaceutical preparations | UV spectrophotometric | DDQ | 494 | methanol | 2.0-30.0 | 0.343 and 1.145 | [72] |
| TCNQ | 841 | Water | 1.5-10 | 0.016 and 0.055 | ||||
| Ninhydrin reagent |
573 | DW | 40-180.0 | 1.235 and 4.117 |
||||
| 3 | Bulk drug and Capsule |
Spectrophotometric | 460 nm | DW | 0.5–7.0 | 0.019 and 0.0647 |
[73] | |
| Spectrofluorimetric | 558 nm | Chloroform | 40–400 ngmL−1 | 0.049 and 0.165 | ||||
| 4 | Bulk, Capsule and in Human Urine Samples | spectrophotometric | 353 nm | DW | 0.5–3.5 | 2.46 × 10−1 8.154 × 10−2 |
[74] | |
| 5 | pure form and in capsules | spectrophotometric method | 402.6 nm | Phosphate buffer pH 7.4 | 50-1000 μg mL-1 | 60 and 200 μg mL-1 | [75] | |
| 6 | Pure drug and pharmaceutical formulation | UV spectrophotometric | 223 nm | Methanol | 2.5-12.5 | 0.31-0.87 | [76] | |
| 7 | Pure form and capsule form | Spectrophotometric | 333 nm | DW | 20-160 | 0.545-1.652 | [77] | |
| Spectrofluorimetric | 470 nm | DW | 0.2-3 | 1.95 × 10-3-5.9*10-3 | ||||
| 8 | Capsule dosage form | spectrophotometric | 365 nm | Water | 2-18 | - | [78] | |
| 9 | Pure form and capsule | spectrophotometric | 385 nm | NaOH solution | 2-10 | 0.24 and 0.74 | [79] | |
Note: Compound: PGB alone in all reported methods.
Table 1: Spectrophotometric method for analysis of PGB.
| Sr. No. | Drug | Matrix | Method | Detection (nm) |
Linearity | LOD and LOQ | Ref |
|---|---|---|---|---|---|---|---|
| 1 | PGB+ MCA+ ALA |
Multi component dosage form |
First order derivative spectroscopic method | 436.24 | 100-140 | 5.0915 and 15.4290 μg/ml | [80] |
| 307.03 | 1-1.4 | 0.01893 and 0.05737 |
|||||
| 383 | 130-170 | 5.4640 and 16.5576 | |||||
| 2 | PGB + PCM | Bulk and tablet formulation. | UV spectroscopic method | 210 | 2-14 | 0.0215 and 0.0651 | [81] |
| 246 | 0.0540 and 0.1638 |
Note: Solvent: water for all the reported methods.
Table 2: Limit of detection and limit of quantification.
| Sr. No | Matrix | Method | Stationary Phase |
Mobile Phase |
Detection (nm) |
FR ml/min | RT | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Bulk, and human urine sample | RP-HPLC | ODS hypersil column (250 mm × 4.6 mm) |
methanol acetonitrile - 0.02 M di - potassium hydrogen orthophosphate (K2HPO4) (pH - 7.00) |
210 | 1.0 | 4.63 | [82] |
| 2 | Pharmaceutical tablet dosage form | RP-HPLC | Phenomenex C18 column (150 × 4.6 mm Id, ODS 2, 5 μm) | 50:50 % (v/v) of MeOHand 10 mM Ammonium Acetate | 210 | 0.7 | 3.39 ± 0.10 | [83] |
| 3 | In bulk/ formulation form | RP-HPLC | Kromasil, C18, 100 × 4.6 mm, 5 μm column | phosphate buffer pH 6.9 and ACN (90:10) | 210 | 1.0 | 4.7 | [84] |
| 4 | In bulk and human urine samples | RP-HPLC | C18 5 μm ODS hypersil column (250 mm × 4.6 mm) |
MeOH:ACN 0.02 M di - potassium hydrogen orthophosphate (K2HPO4) (pH - 7.00) |
210 | 1.0 | 3.9 | [85] |
| 5 | Human plasma | HPLC | TRACER EXCEL ODS-A stainless steel column, (5 μm, 150 × 4.6 mm |
Methanol and Na2HPO4 (65:35) |
360 | 1.0 | - | [86] |
| 6 | In bulk drug, and human serum has | RP-HPLC | KROMASIL 100-5 C-18 column (250×4.6 i.d. mm) | buffer pH 7 and ACN (96:4, v/v) |
210 | 1.0 | 4.6 | [87] |
| 7 | Bulk drugs and in capsule dosage forms. | HPLC | Inertsil ODS -3V, C18 (250 × 4.6 mm Id, 5 μm) column | 80: 10: 10 (v/v/v) of Disodium Hydrogen Phosphate Buffer: ACN: MeOH. |
210 | 1.0 | 4.7 | [88] |
| 8 | Pharmaceutical and bulk formulation | RP-HPLC | C18 5 μm BDS hypersil column (250 mm × 4.6 mm) |
phosphate buffer solution (pH 6.9) and acetonitrile (94:6) |
210 | 1.0 | 9.0 | [89] |
Note: Compound: PGB is used as drug sample in all reported methods
Table 3: HPLC methods for analysis of PGB.
These reported methods describe the evaluation of PGB in the various dosage forms in a single constituent and in combination with GBP, MCA, Paracetamol (PCM), Methylcobalamin, Vigabatrin, Sildenafil, Amitriptyline spectacles different analytical method carries out for estimation of PGB. With the combination of four second-generation antiepileptic drugs, including PGB, GBP, VGB, and Topiramate, the analysts were elicited from blood plasma by the help of extensive solid- phase extraction derivatized with 4-chloro-7-nitrobenzofurazan and detection of HPLC with fluorescence detection. The scheme is confirmed acceptable for all four analysts and relevant for daily use [97-104]. Currently, the use of LC-MS/MS [105-107] has been gaining interest due to the high levels of analytical sensibility achieved and the lower limit of detection that this tool is able to provide for drug quantification and identification (Tables 2 and 3).
Spectrophotometric methods
To date, numerous spectroscopic methods have been accounted for the determination of PGB sole and in combination. The present review highlights the analytical methods used for the quantification and identification of PGB in pharmaceuticals and biological samples, particularly focusing on the HPLC-based methods. Even though the clinical use of PGB has been approved for a long, but the active participation of enantiomers LYRICA®22(2005), synthesis, and separation of enantiomers remains keen interesting points for the study of PGB. Therefore, the present review describes only those analytical methods that were published in the scientific literature for PGB since its discovery. The analytical studies of PGB were done in bulk and pharmaceutical formulation which involves several calculations of absorbance ranges starting from 210 nm [71]. In research, the addition of the chromophoric group for UV spectroscopy was made through the reaction of benzoylation to give a sensitive derivative of PGB [76]. From the study of Armagan and co-workers, it was found that PGB acts as anelectron donor with the DDQ and TCNQ as п acceptors which give extremely colored compounds at 494 and 841 nm. Here TCNQ was found to be more preferable based on higher molar absorptivity and lower detection limit [72]. The Spectro-fluorometric method of PGB with sensitive fluorogenic and chromogenic indicator 7-chloro-4-nitrobenzofurazon was used for routine quality control analysis without the intrusion of excipients [73]. In another study, by collecting a biological sample (Human Urine) by RS Gujral and co-workers, the PGB was blended with potassium iodate and potassium iodide to provide excellent accuracy and precision. It may assist in determining the influence of this drug on a human being meanwhile the treatment [74].
Chromatographic overview
In the widely used methods of spectroscopy like RP-HPLC for Isocratic elusion, hypersil C18 column (250 mm × 4.6 mm) was used. The combined mobile phase system like methanol: acetonitrile: potassium hydrogen orthophosphate (3:1:16v/v/v) [82] and potassium dihydrogen orthophosphate buffer (balanced for pH 6 by utilizing NaOH solvent): acetonitrile: methanol (75:10:15v/v/v) [94] were used for very easy, cost-efficient, quick, and effective method development. The main benefit of this method involves short retention time, without depletion with another reagent, the stability of solvent, no requirement for the earlier separation and purification. The less chromatographic time creates this method appropriate for the processing of numerous samples indefinite periods of time. In another estimation of PGB in human plasma, it was concluded that the method based on the derivatization of PGB with FDED in alkaline solution provides satisfactory results. The colored product can be found by a UV detector at less concentration [86].
HPTLC method
Another sensitive and reliable spectroscopy after HPLC is the HPTLC method. According to Patil and co-workers the concurrent determination of PGB and Aceclofenac stability-indicating method, chromatographic departure was carried out on aluminum plate smear with silica gel 60 F254, and the mobile phase was selected as toluene: methanol: formic acid (7:3:0.2v/v/v). In this method, all parameters were met with acceptable standards [95,96]. In another method, PGB and amitriptyline hydrochloride estimation was done with densitometry in pharmaceutical preparations. The silica gel 60F254 was used as a static phase and for the mobile phase toluene, methanol, and formic acid (7:2.5:0.5v/v/v) are used. This scheme concludes that the established method has many benefits like less cost consuming, relatively fast, stable, distinct, and easily reproducible [97,98] (Table 4).
| Drug | Matrix | Method | Stationary phase | Mobile phase | Detection (nm) |
Rf | Ref |
|---|---|---|---|---|---|---|---|
| Aceclofenac +PGB | In bulk and in formulation | stability-indicating HPTLC | Silica Gel 60 F254 HPTLC Plate | Toluene: MeOH: Formic acid (7: 3: 0.2 v/v/v) | 210 | 0.68 ± 0.03 (ACF)and 0.27 ± 0.03(PGB) | [95] |
| PGB + Amitriptyline HCl | Pharmaceutical dosage form | HPTLC Densitometry |
Silica gel 60 F254 HPTLC method | Toluene: Methanol: Formic acid (7: 2.5: 0.5 v/v/v) | 205 | 0.27 ± 0.03(PGB) 0.68 ± 0.03(AMTR) | [96] |
| GBP+ PGB | Pharmaceutical dosage forms. | HPTLC method | Silica Gel G60 F254 | Ethyl Acetate: MeOH: Ammonia (6.0: 4.0: 0.1 v/v) | 210 | 0.993 (GBP) 0.992 (PBG) |
[97] |
| Milnacipran HCl+ Duloxetine HCl+ PGB |
Bulk drug and pharmaceutical formulation | Staility indicating HPTLC method | Silica gel 60 F254 | ACN-water-ammonia (6:0.6:1.6, v/v/v) | 220 | 0.9996 | [98] |
| dichloromethane-MeOH(8:1, v/v) | 230 | 0.9995 | |||||
| ethyl acetate-MeOH: ammonia (6:3:0.1, v/v/v) | 210 | 0.9999 |
Table 4: HPTLC methods for analysis of PGB in combination.
LC-MS/MS method
Currently, the LC-MS and LC-MS/MS is the adaptable analytical tool that blends liquid chromatography resolute strength with mass spectrometry detection specificity. Sample compounds are isolated by liquid chromatography and then added to the mass spectrometer. The mass spectrometer generates the charge ions which then tracked [99-102]. The estimation of PGB alone and in combination is shown in Tables 5 and 6 which includes different parameters like stationary phase, mobile phase, detector, internal standard, etc. The research of N. Kostic and co-workers include the determination ofPGB by novel LC-MS method in the dried matrix sport (DMS) [99-108] (Table 5).
| Sr No | Stationary phase | Mobile phase | Detection\ Detector |
IS | FR | Ref |
|---|---|---|---|---|---|---|
| 1 | YMC-Pack Octyl column (50 × 4.0 mm, 3 μm particle size) | ACN: 0.15% formic acid (85:15, v/v). | A TSQ Quantum 104 Access MAX triple quadrupole |
- | 550 μL/min | [99] |
| 2 | Kromasil 100 C18 (3.5 μm, 3, 30 mM) column | ACN-0.5% formic acid (80:20) | triple quadrupole mass spectrometer | Tramadol | 1 mL/min |
[100] |
| 3 | Hypurity, 5 mm C-18 (50 ´ 4.6 mm i.d.) | buffer-MeOH 20:80 (v/v) | Biosystems MDS Sciex (API 2000) |
Imipramine | 0.9 mL/min | [101] |
| 4 | Waters Symmetry® C18, 100 mm × 4.6 mm, 3.5 m |
formic acid and ACN (30:70, v/v) | API 4000 triple quadrupole instrument |
Rosuvastatin | 1.0 mL/min | [102] |
| 5 | Shiseido Capcell Pak MG C18 column |
ammonium acetate and ACN (15:85, v/v) | API 2000 | Losartan | 0.2 mL/min | [103] |
| 6 | ThermoHypurity C18 5 lm analytical column | ACN a2 mM ammonium acetate 80:20 (v/v) | API 2000 instrument | _ | 1.0 mL/min | [104] |
Note: Method applied: LC-MS/MS and Matrix: Human Plasma for all reported methods
Table 5: LC-MS/MS methods for analysis of PGB.
The appealing method of sample accumulation in micro quantity was utilized in the form of dried blood spot (DBS) and dried plasma spot (DPS) followed by the pre-column derivatization method [109-115]. From the analysis, it was concluded that the DPS is certainly can become an appropriate component for all parameters using a plasma matrix. Nevertheless, the potential depreciation of plasma by DBS depends on overcoming the hematocrit issue[116-119] (Table 6).
| Sr No | Drug | Matrix | Stationary Phase |
Mobile phase |
Detection/ detector |
FR mL/min |
Ref |
|---|---|---|---|---|---|---|---|
| 1 | PGB Sildenafil and Its Active Metabolite | Rat plasma | Chromolith Speed Rod RP-18e, 50 9 4.6 mm Column |
10:90 (v/v) MeOH:water | API 3000 Triple Quadrupole |
3 | [105] |
| 2 | PGB and GBP | In urine | PhenomenexKinetex, 2.1 mm 50 mm 2.6 μm | 1:1:1 MeOH:ACN:water (v/v) | AB SCIEX API 4000 | 0.4 | [106] |
| 3 | BLF, GBP and PGB | human post-mortem blood | Poroshell 120 EC-C18 HPLC column | Mobiphase A: deionized water:formic acid (0.1%). Mob phase B: ACN: Formic acid (0.1%). |
Agilent 6460 Triple Quadrupole mass spectrometer |
- | [107] |
Note: Method applied: LC-MS/MS for all reported methods
Table 6: LC-MS/MS methods for analysis of PGB in combination.
GC and GC/MS method
Yet another interesting method for detection of the PGB is GC and GC/ MS method. Theuniqueness in chemical structure (presence of acidic and basic functional group), provides amphoteric physicochemical properties to PGB results in selective solubility. PGB being amphoteric shows free solubility in water, but sparingly in methanol and ethanol [120]. The presence of a chiral center (Fig. 1) suggests chiral derivatization of its amine moiety, which is yet another challenge because of zwitterionicproperties of PGB which prevents convectional GC-MS methods. The zwitterionic structure (Fig. 9) of PGB shows the negative charge on active site (carboxylic acid), results poor chromatography inGC-MS (Fig. 9).
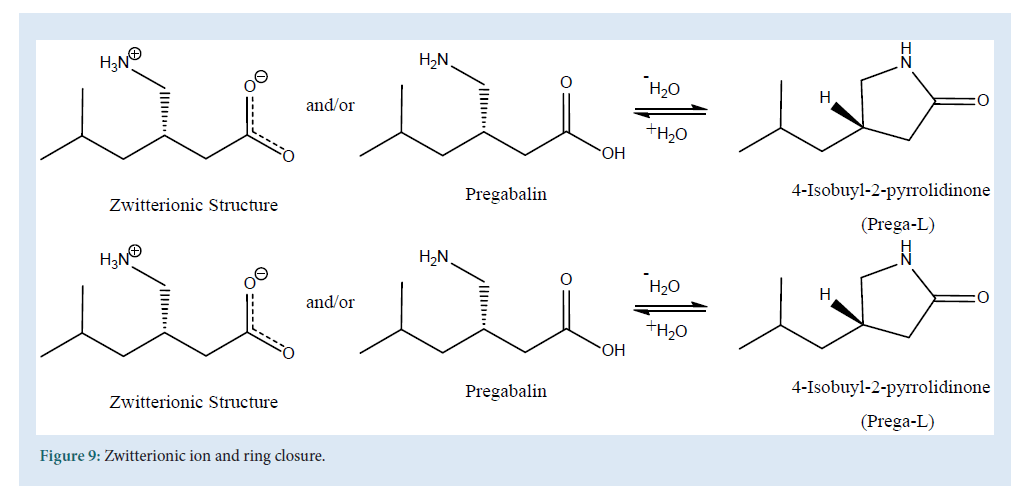
Figure 9: Zwitterionic ion and ring closure.
Moreover, the dehydration of PGB, due to high temperature in GC-MS, may result in formation of Prega-L or 4-isobutyl-2-pyrrolidinone or lactam (Fig. 9) which is considered as a highly toxic substance. Determination of PGB via GC and GC-MS methods are listed in Table 7 . Generally the ethyl chloroformate (ECF) was used in several GC-MS methods as derivatizing reagent, but the use of newer agents like (S)-TPC [120], IBCF, DCC and NHS also provides us very satisfactory results during estimation of PGB in different matrices like urine, blood and human plasma (Table 7).
| SrNo | Matrix | Method | Model number | Internal Std | Column | Derivatizing reagent |
Injector Temp °C |
Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Bulk Drug | GC-MS | Agilent – 7693/ GC-MS (7890B/5977A) | - | 30 m, 250 µm i.d., 25 µm film thickness | (S) trifluoroacetylprolyl chloride or (S)-TPC |
280 | [120] |
| 2 | Tissues and Blood | GC-MS | Agilent 6890 | Ibuprofen | HP-5-MS; 0.25 mm × 60 m × 0.25 um capillary, 60 m × 250 µm | - | - | [121] |
| 3 | Urine Sample | GC-MS | GC Ultra with Triplus autoSampler +TSQ Quantum XLS Mass spectrometer | - | Elite-5 MS 60 m × 0.25 µm film thickness | ECF+MCF+ IBCF |
270 | [122] |
| 4 | Human Plasma | GC | Shimadzu -2014, Japan | - | Rtx-5 cross bond 30 m × 0.25 µm | ECF | 170 | [123] |
| 5 | Bulk drug and Tablet | GC | Shimadzu-2014, Japan | - | Rtx-5 cross bond 30 m × 0.25 µm | ECF | 170 | [124] |
| 6 | Human Plasma | GC-MS | Agilent- 7890 B | GBP | 30 m × 0.25 µm | EDC+ NHS+ DCC |
280 | [125] |
Note: Drug: PGB is used for all reported methods
Table 7: GC/GC-MS methods for analysis of PGB.
The method of protection of carboxylic acid groupconverting to methyl ester [120], Salt induces precipitation, micro-extraction techniques (solid-phase micro-extraction and dispersive liquid–liquid micro-extraction) are listed in Table 7.
PGB is a potent ligand of alpha-2-delta subunit of voltage-gated calcium channel in the central nervous system which shows analgesic, anti- convulsant and anxiolytic activity. It is a structural analog of GABA (γ-aminobutyric acid) but functionally dissimilar to naturally occurring transmitter. Although the drug received approval for since long, but, due to highly active enantiomeric form generation, only a few analogs are able to reach till stages of clinical development. The unique mechanism of action renders PGB substantially important for medical and clinical use. Furthermore, PGB exhibits suitable pharmacokinetic properties and high stability with a creation of negligible amount (2%) of metabolites. Considering zwitterionic properties and solubility in water, the HPLC-based methods are widely preferred for estimation of PGB as the ring closure does not occur at room temperature. Several other coupled methods like UV, LC-MS/MS, or mass spectroscopy, are the major analytical techniques available in the literature for the determination of PGB in bulk and biological samples. The manuscript is being designed on considering the recent data available (for 10 years). The pharmacokinetic, mechanism of action and synthetical part may be older than selected years but the compiled spectroscopical methods are from 2010-2021 with the consideration of the patent of LYRICA (2005). The advantages of the methods based on all spectrometers may be attributed to accuracy and sensitivity with high specificity which can be determined by these methods. Critically applied methods like LC-MS/MS and HPTLC are also mentioned enantiomeric separation of PGB. The presence of a chiral group and ring closure due to high temperature suggests the use of selected chiral derivatizing agents in GC and GC-MS, which are compiled in the present review. The present review elaborates various analytical approaches exercised for the appraisal of PGB and the current scenario to apply the state of the art of analytical development. Further methods are reported for its pharmacokinetics as well as bioequivalence studies.
Ethics approval and consent to participate: Not Applicable
Consent for publication: Not Applicable
Availability of data and material: The data, if required, will be available upon request
Competing interests: The authors declare no competing financial interest
Not Applicable
SB, KA and US contributed equally for preceding this research. Concept and guidance was from AS and final manuscript was prepared and checked by AN and PS. We declare that all authors have read and approved the manuscript before submission.
The authors like to thank the Department of Science and Technology- FIST, Govt. of India (Grant No. SR/FST/College-2018/268) for (HPTLC) instrument funding. We would also like to thank Principal, Head of Department and In-charge of the Central Instrument Lab (CIF) for guidance.
[Google scholar], [Indexed]
[Indexed]
[Cross ref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Google scholar], [Indexed].
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Google scholar], [Indexed].
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Google scholar], [Crossref], [Indexed].
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed].
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]
[Crossref], [Google scholar]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar], [Indexed]
[Crossref], [Google scholar]